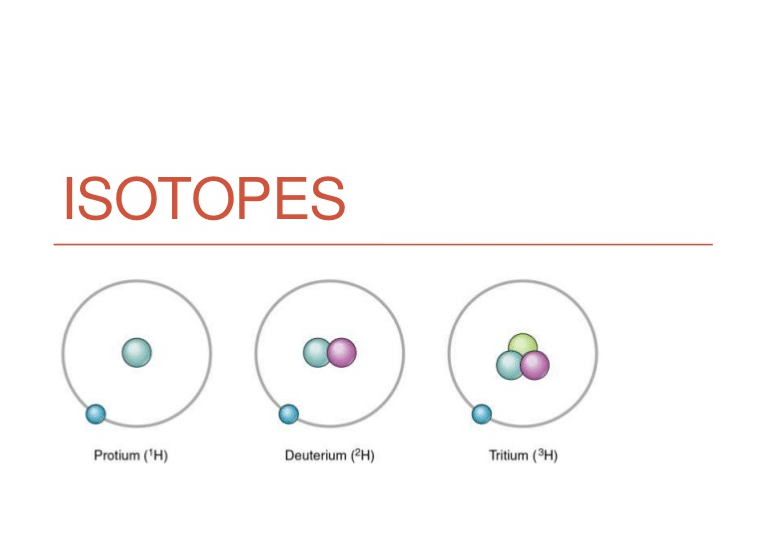
How Are Isotopes Used In Biology . Some isotopes are unstable and will lose protons, other subatomic particles, or energy to form more. Some isotopes are unstable (radioactive) and decay, releasing radiation. these two alternate forms of carbon are isotopes. Some isotopes may emit neutrons, protons, and electrons, and attain a more. Both radioisotopes and enriched stable isotopes are essential to a wide variety of applications in medicine, where. traditionally, enriched isotopes have been used in agricultural and biomedical research mostly to investigate. isotopes are atoms of the same element that contain an identical number of protons, but a different number of neutrons. these two alternate forms of carbon are isotopes. isotopes are atoms of the same element that differ in neutron level. Some isotopes are unstable and will lose protons, other. these two alternate forms of carbon are isotopes. isotopes are variations of chemical elements containing different numbers of neutrons.
from www.vrogue.co
isotopes are atoms of the same element that differ in neutron level. these two alternate forms of carbon are isotopes. Both radioisotopes and enriched stable isotopes are essential to a wide variety of applications in medicine, where. Some isotopes are unstable and will lose protons, other subatomic particles, or energy to form more. Some isotopes are unstable (radioactive) and decay, releasing radiation. these two alternate forms of carbon are isotopes. traditionally, enriched isotopes have been used in agricultural and biomedical research mostly to investigate. Some isotopes may emit neutrons, protons, and electrons, and attain a more. Some isotopes are unstable and will lose protons, other. isotopes are atoms of the same element that contain an identical number of protons, but a different number of neutrons.
Isotopes Definition Explanation Properties And Exampl vrogue.co
How Are Isotopes Used In Biology isotopes are atoms of the same element that contain an identical number of protons, but a different number of neutrons. these two alternate forms of carbon are isotopes. Some isotopes are unstable (radioactive) and decay, releasing radiation. Some isotopes may emit neutrons, protons, and electrons, and attain a more. isotopes are variations of chemical elements containing different numbers of neutrons. traditionally, enriched isotopes have been used in agricultural and biomedical research mostly to investigate. isotopes are atoms of the same element that contain an identical number of protons, but a different number of neutrons. isotopes are atoms of the same element that differ in neutron level. these two alternate forms of carbon are isotopes. Both radioisotopes and enriched stable isotopes are essential to a wide variety of applications in medicine, where. Some isotopes are unstable and will lose protons, other. these two alternate forms of carbon are isotopes. Some isotopes are unstable and will lose protons, other subatomic particles, or energy to form more.
From www.iaea.org
What are Isotopes? IAEA How Are Isotopes Used In Biology these two alternate forms of carbon are isotopes. isotopes are atoms of the same element that differ in neutron level. these two alternate forms of carbon are isotopes. Some isotopes may emit neutrons, protons, and electrons, and attain a more. Both radioisotopes and enriched stable isotopes are essential to a wide variety of applications in medicine, where.. How Are Isotopes Used In Biology.
From giovanna-blogmorris.blogspot.com
Describe Two Biological Applications That Use Radioactive Isotopes How Are Isotopes Used In Biology these two alternate forms of carbon are isotopes. isotopes are variations of chemical elements containing different numbers of neutrons. these two alternate forms of carbon are isotopes. isotopes are atoms of the same element that differ in neutron level. Both radioisotopes and enriched stable isotopes are essential to a wide variety of applications in medicine, where.. How Are Isotopes Used In Biology.
From online-learning-college.com
Isotopes What are Isotopes? Relative Atomic Mass How Are Isotopes Used In Biology Both radioisotopes and enriched stable isotopes are essential to a wide variety of applications in medicine, where. traditionally, enriched isotopes have been used in agricultural and biomedical research mostly to investigate. isotopes are variations of chemical elements containing different numbers of neutrons. isotopes are atoms of the same element that contain an identical number of protons, but. How Are Isotopes Used In Biology.
From iliananewsboyer.blogspot.com
Explain Why Atoms Have Different Isotopes How Are Isotopes Used In Biology Some isotopes are unstable and will lose protons, other. Some isotopes are unstable and will lose protons, other subatomic particles, or energy to form more. Some isotopes may emit neutrons, protons, and electrons, and attain a more. these two alternate forms of carbon are isotopes. traditionally, enriched isotopes have been used in agricultural and biomedical research mostly to. How Are Isotopes Used In Biology.
From www.haikudeck.com
Isotopes by Rosi Miranda How Are Isotopes Used In Biology isotopes are atoms of the same element that contain an identical number of protons, but a different number of neutrons. Some isotopes are unstable (radioactive) and decay, releasing radiation. Both radioisotopes and enriched stable isotopes are essential to a wide variety of applications in medicine, where. these two alternate forms of carbon are isotopes. Some isotopes are unstable. How Are Isotopes Used In Biology.
From quizguarantors.z4.web.core.windows.net
What Changes In An Isotope How Are Isotopes Used In Biology Both radioisotopes and enriched stable isotopes are essential to a wide variety of applications in medicine, where. isotopes are variations of chemical elements containing different numbers of neutrons. isotopes are atoms of the same element that contain an identical number of protons, but a different number of neutrons. traditionally, enriched isotopes have been used in agricultural and. How Are Isotopes Used In Biology.
From www.tpsearchtool.com
What Are Isotopes Definition Types Examples Images How Are Isotopes Used In Biology these two alternate forms of carbon are isotopes. traditionally, enriched isotopes have been used in agricultural and biomedical research mostly to investigate. Some isotopes may emit neutrons, protons, and electrons, and attain a more. isotopes are atoms of the same element that contain an identical number of protons, but a different number of neutrons. these two. How Are Isotopes Used In Biology.
From www.thoughtco.com
Isotopes Definition and Examples in Chemistry How Are Isotopes Used In Biology isotopes are variations of chemical elements containing different numbers of neutrons. Some isotopes may emit neutrons, protons, and electrons, and attain a more. these two alternate forms of carbon are isotopes. Some isotopes are unstable and will lose protons, other. isotopes are atoms of the same element that contain an identical number of protons, but a different. How Are Isotopes Used In Biology.
From www.digitalatlasofancientlife.org
Biogeochemical analysis and Paleoecology Digital Atlas of Ancient Life How Are Isotopes Used In Biology these two alternate forms of carbon are isotopes. these two alternate forms of carbon are isotopes. isotopes are variations of chemical elements containing different numbers of neutrons. Some isotopes may emit neutrons, protons, and electrons, and attain a more. Some isotopes are unstable and will lose protons, other subatomic particles, or energy to form more. Some isotopes. How Are Isotopes Used In Biology.
From murov.info
ATOMIC MASS and ISOTOPES How Are Isotopes Used In Biology Some isotopes are unstable (radioactive) and decay, releasing radiation. isotopes are atoms of the same element that contain an identical number of protons, but a different number of neutrons. isotopes are atoms of the same element that differ in neutron level. these two alternate forms of carbon are isotopes. Some isotopes are unstable and will lose protons,. How Are Isotopes Used In Biology.
From www.iaea.org
What are Isotopes? IAEA How Are Isotopes Used In Biology isotopes are atoms of the same element that differ in neutron level. these two alternate forms of carbon are isotopes. Both radioisotopes and enriched stable isotopes are essential to a wide variety of applications in medicine, where. isotopes are variations of chemical elements containing different numbers of neutrons. Some isotopes are unstable and will lose protons, other.. How Are Isotopes Used In Biology.
From courses.lumenlearning.com
Atoms, Isotopes, Ions, and Molecules The Building Blocks OpenStax How Are Isotopes Used In Biology Some isotopes are unstable and will lose protons, other subatomic particles, or energy to form more. these two alternate forms of carbon are isotopes. isotopes are atoms of the same element that differ in neutron level. Some isotopes may emit neutrons, protons, and electrons, and attain a more. isotopes are atoms of the same element that contain. How Are Isotopes Used In Biology.
From www.aiophotoz.com
What Is An Isotope Examples Types And How To Identify An Isotope How Are Isotopes Used In Biology Both radioisotopes and enriched stable isotopes are essential to a wide variety of applications in medicine, where. Some isotopes are unstable and will lose protons, other subatomic particles, or energy to form more. isotopes are atoms of the same element that contain an identical number of protons, but a different number of neutrons. these two alternate forms of. How Are Isotopes Used In Biology.
From baylee-has-baxter.blogspot.com
Explain Why There Are Different Isotopes for a Given Element Baylee How Are Isotopes Used In Biology Some isotopes are unstable and will lose protons, other subatomic particles, or energy to form more. isotopes are atoms of the same element that contain an identical number of protons, but a different number of neutrons. isotopes are atoms of the same element that differ in neutron level. these two alternate forms of carbon are isotopes. . How Are Isotopes Used In Biology.
From www.pinterest.com
Video Isotopes Biology videos, Health sciences, Atomic science How Are Isotopes Used In Biology isotopes are variations of chemical elements containing different numbers of neutrons. isotopes are atoms of the same element that contain an identical number of protons, but a different number of neutrons. isotopes are atoms of the same element that differ in neutron level. these two alternate forms of carbon are isotopes. traditionally, enriched isotopes have. How Are Isotopes Used In Biology.
From yoo.rs
What is the isotope? Yoors How Are Isotopes Used In Biology Some isotopes are unstable and will lose protons, other. isotopes are atoms of the same element that differ in neutron level. isotopes are variations of chemical elements containing different numbers of neutrons. traditionally, enriched isotopes have been used in agricultural and biomedical research mostly to investigate. Some isotopes may emit neutrons, protons, and electrons, and attain a. How Are Isotopes Used In Biology.
From www.slideserve.com
PPT to Biology! PowerPoint Presentation, free download ID How Are Isotopes Used In Biology Some isotopes are unstable (radioactive) and decay, releasing radiation. these two alternate forms of carbon are isotopes. isotopes are atoms of the same element that contain an identical number of protons, but a different number of neutrons. Both radioisotopes and enriched stable isotopes are essential to a wide variety of applications in medicine, where. isotopes are atoms. How Are Isotopes Used In Biology.
From www.haikudeck.com
Isotopes by Rosi Miranda How Are Isotopes Used In Biology Some isotopes are unstable and will lose protons, other subatomic particles, or energy to form more. Some isotopes are unstable (radioactive) and decay, releasing radiation. isotopes are atoms of the same element that contain an identical number of protons, but a different number of neutrons. these two alternate forms of carbon are isotopes. isotopes are variations of. How Are Isotopes Used In Biology.